Life History & Behaviour
FEEDING
There is considerable confusion surrounding the diet of M. rosea. As this species has been placed into the Tritoniidae family of Nudibranchia, observed feeding patterns do not reflect those of nudibranchs belonging to this family. Tritoniids are known to feed exclusively upon soft corals and gorgonians, while M. rosea has been observed feeding upon arborescent hydroid species, which is the primary food source of nudibranchs belonging to the Aeolididae family (Willan & Coleman, 1984). This species has also been identified foraging on zoanthid colonies, which raises questions as to what the primary food source of M. rosea actually is.
All nudibranchs are carnivorous predators and consume their prey through the use of a specialised feeding apparatus known as the radula. This highly evolved feeding apparatus is located inside the buccal cavity of the mouth. This tongue-like apparatus is lined with thousands of tiny razor-sharp chitinous teeth, arranged in lateral rows inside the buccal cavity (Ruppert et al. 2004).
Nudibranch eyes are of little use to the animal, which is why these animals have evolved the use of chemosensory rhinophores and oral tentacles that allow nudibranchs to locate food through olfaction. Often observed as small pigment spots on top of the animal’s head, they eyes are actually located deep inside the body on top of the brain (Rudman 2000). Nudibranch eyes are unable to make out distinct images, but can differentiate between light and dark. The primary use of eyes in nudibranchs may be to detect shadows of predators, and to keep the animal’s internal body clock timed to a 24 hour day and night cycle (Rudman 2000). In M. rosea, the length of the optic nerve is significantly reduced, an anatomical feature shared with those species belonging to Aeolidiidae (Willan 1988).
The rhinophores of M. rosea are associated with smell, and can sense chemicals in the surrounding water column. The oral tentacles are considered to be associated with touch, and aid the nudibranch in feeling its way around substrates and locating food sources (Rudman 2000). All nudibranchs have scent receptors located on their rhinophores that pick up scents in the water column. M. rosea is specialised in that it has papillae present in the rhinophoral structure that provide a greater surface area to test for chemicals in the water (Rudman 2000).
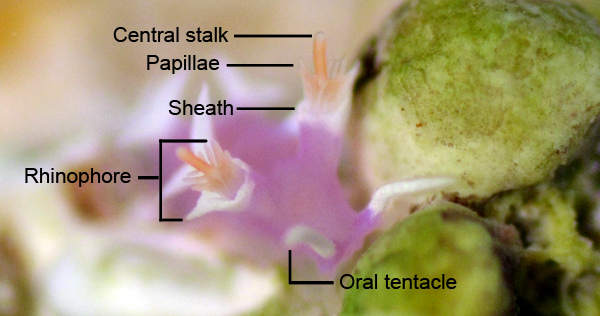
Through the use of extendable protractor muscles, the protruding radula is used to scrape food material off the substrate and direct food through the buccal cavity and towards the digestive cavity where the material is digested. As the nudibranch scrapes its radula back and forth over the substrate, food particles are captured in the chitinous teeth and transported to the oesophagous through a conveyor-belt like movement (Rudman 2000). The morphology and structure of the buccal cavity in Tritoniids is characteristic to species of this family. The anatomy of M. rosea is further explored under the Anatomy and Physiology section.
EXCRETION
All nudibranchs expel their waste matter through the anus, which is located on the right lateral side of the posterior end of the animal. Food is digested in the stomach and waste transported to the intestine. Waste pallets are then passed through the lateral anal site, and expelled from the body of the nudibranch through the distinct renal pore opening (Rudman 2000).
RESPIRATION
All nudibranchs acquire oxygen through the use oftheir ‘naked gills’ or branchial plumes, which are generally exposed on the outside of the body (Debelius & Kuiter 2007). Each species of nudibranch has a unique way of obtaining oxygen from the water column through these specialised gills. Marianina rosea respires through its bifurcated cerata that contains these ‘naked gills’. The branched arrangement of cerata in Dendronotaceans have evolved to increase the surface area that is available for gas exchange (Picton & Morrow, 1994). The epidermal layer of the cerata is thin enough to allow gas exchange to occur across the membrane, as blood absorbs the oxygen from the water column (Behrens 2005). Oxygen is both taken up and lost through the cerata of this species. This method of respiration is attributable to members of the Tritoniidae.

LOCOMOTION
Marianina rosea propels itself along the seafloor by using its highly evolved muscularised foot, located on the underside of the mantle. When placed on a piece of coral boulder, the muscularised foot adheres to the substratum allowing the muscularised foot to glide along the surface. The foot exhibits two specialised muscular bands; a thicker outer band that is kept in constant contact with the substratum and an elongate inner strip of tissue, known as the sole. The sole produces waves of muscular contractions that pull the slug forward (Behrens 2005). Specialised pedal glands produce a sticky mucous allowing the animal to securely adhere to any surface. The muscular foot is also coated in a layer of fine cilia that aid in locomotion. Movement of M. rosea is relatively slow, however if the nudibranch becomes dislodged it will immediately engage in swimming to find a new surface. This method of locomotion is not effective in mature M. rosea, but may be an effective dispersal method for juveniles to colonise new environments.

REPRODUCTION
All species belonging to Nudibranchia are hermaphroditic. Most nudibranchs have employed mechanisms to avoid self-fertilisation, however the specific mechanisms are unclear (Wells & Bryce, 1993). Mature specimens display both male and female reproductive organs, which are positioned together and collectively referred to as the gonad. In Marianina rosea mating is opportunistic with copulation occurring between two individuals, of which each can act as both male and female. The individuals will position themselves in a head to tail formation, with right sides touching and exchange sperm as the penis of each individual enters the female organ. Upon completion of fertilisation, the animals disperse and both are capable of depositing their spawn on a suitable substrate (Behrens 2005). Anticlockwise spiral egg ribbons of M. rosea are often found on the axes o fhydroid branches, their primary food source (Willan & Coleman, 1984).
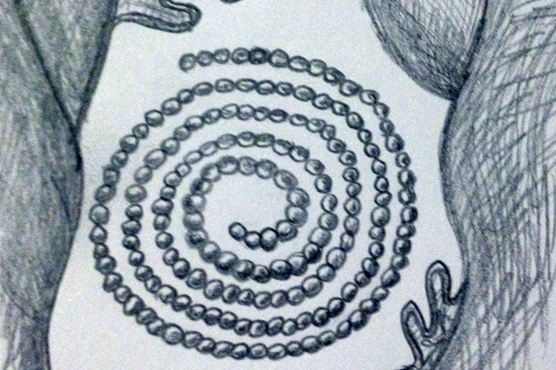
Depiction of white egg-ribbon spiral on substrate (sketch by Elisha Simpson 2013).
These eggs then hatch into planktonic veliger larvae. At this veliger stage, all larvae have the chitinous shell that is characteristic of their class. This shell is lost over developmental time as the larvae settles to the benthos and metamorphoses into a juvenile (Behrens 2005). Juveniles then develop and grow into sexually mature adults. The lifecycle of a nudibranch is considerably short. Many species will not live beyond one year, with some species’ lifespan lasting only weeks. Nudibranchs are considered to be highly fecund animals, however, M. rosea are only found in abundance when food availability is high (Willan & Coleman, 1984). Many individuals will never find a mate and reproduce in their lifetime, making it extremely difficult to locate populations on reefs, as they are an uncommon species.
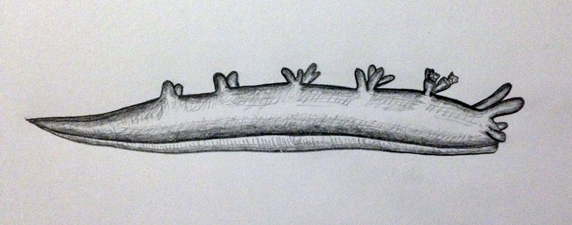 Juvenile depiction with undeveloped external morphology (biological drawing by Elisha Simpson 2013).
Juvenile depiction with undeveloped external morphology (biological drawing by Elisha Simpson 2013).
BEHAVIOUR
Two distinct behaviours of M. rosea were observed at Heron Island; the distinctive swimming behaviour in this species and another behaviour known as rearing.
SWIMMING
If the threat arises, most nudibranchs can dislodge themselves from a substrate and swim away from potential predation. When disturbed M. rosea dislodges itself from a substrate and swims by flexing its body through the water until it reaches a new substrate. The animal undergoes a left to right flexing motion observed in other Tritoniid species, that occurs when longitudinal muscles along each side of the body contract alternately (Newcomb et al. 2012). This swimming behaviour is considerably slow, although effective enough for the animal to make use of its flexible muscular body and propel itself through the water column.

REARING
Another behaviour was observed in M. rosea upon collection of the animal. The individual was noticed undergoing an unusual behaviour known as ‘rearing’. The animal would lift its upper body from the substrate and survey the surrounding environment with its oral tentacles and rhinophores waving profusely in the water. This behaviour is often observed in nudibranchs that are rearing away from spiculated sponges or stinging corals (Behrens 2005). It is, however, more likely that the animals are simply adjusting the position of their bodies to sense the location of a suitable food source or potential mate.
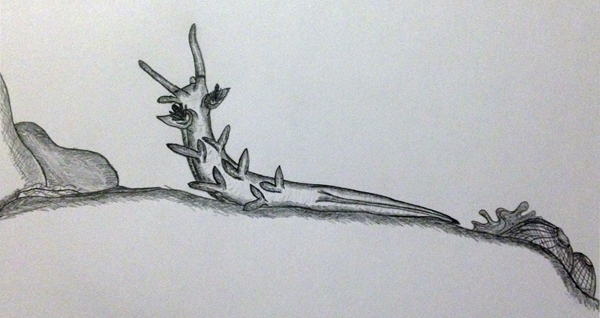
Depiction of rearing behaviour (sketch by Elisha Simpson 2013).
|